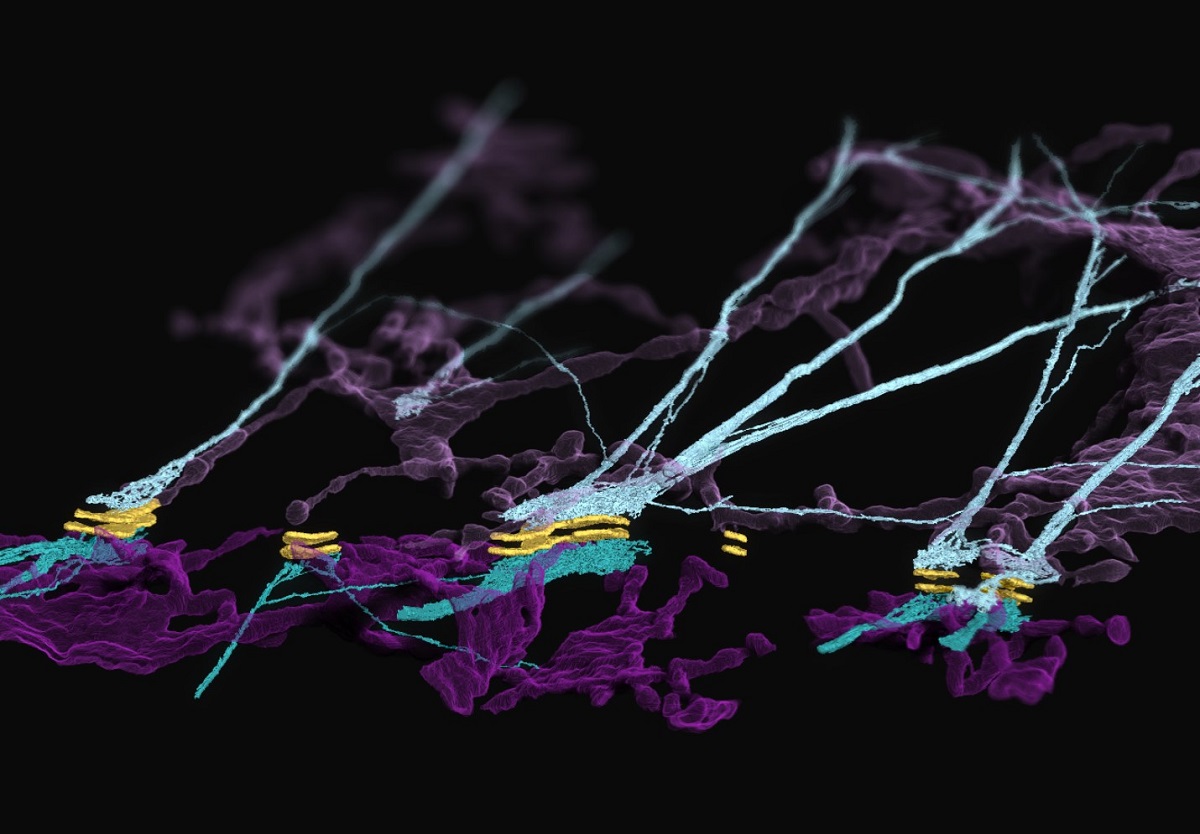
 Penn State College of Medicine researchers used focused ion beam scanning electron microscopy to reveal a previously unknown complex formed at cell-to-cell junctions consisting of desmosomes (yellow), keratin filaments (teal) and endoplasmic reticulum (purple).
Penn State College of Medicine researchers used focused ion beam scanning electron microscopy to reveal a previously unknown complex formed at cell-to-cell junctions consisting of desmosomes (yellow), keratin filaments (teal) and endoplasmic reticulum (purple).
College of Medicine team discovers new molecular complex at cellular junctions
Penn State College of Medicine researchers are using advanced imaging techniques to study how life functions at the atomic, cellular, tissue and organism levels. These research projects are driven by diseases observed in clinic, and the discoveries scientists are making in the lab could someday lead to new treatments for patients with skin diseases, cancers, neurological conditions and other disorders.
October 19, 2023
One of these projects is led by Andrew Kowalczyk, PhD, professor of dermatology and of cellular and molecular physiology, who uses rare skin diseases as the context for studying how cells adhere to each other, or form connections. Scientists estimate there are more than 30 trillion cells in the human body, and the ability for these cells to connect to form tissues and respond to stress is essential for normal tissue and organ function.
“Rare diseases affect few people, but they can reveal so much about fundamental biology that we take for granted,” said Kowalczyk, the Department of Dermatology Endowed Professor. “Our lab’s goal is to make critical discoveries about cell function in the context of rare diseases that can be applied to a multitude of biomedical problems.”
Kowalczyk’s team consists of postdoctoral scholars, graduate students, staff, faculty and international collaborators. With their combined expertise in live cell imaging, machine/deep learning and other techniques, the team aims to make fundamental discoveries about contacts between human cells.
“We’re bringing the brightest minds from multiple scientific disciplines together to answer fundamental questions about how living cells function at the molecular level. Our faculty, staff and trainees are making discoveries that will shape the future of medicine.” – Leslie Parent, MD, vice dean for research and graduate studies
Teamwork and imaging expertise lead to an unexpected discovery
In particular, the Kowalczyk lab studies desmosomes, which are cellular adhesion structures essential to the function of organs like the skin and the heart. In their latest work, published in Nature Cell Biology, the researchers described the architecture and dynamics of a complex formed between desmosomes and the endoplasmic reticulum (ER) — a membranous system within cells involved in protein folding, modification and transport. They made what they called an “unexpected” discovery in the cellular junctions: the ER associates with desmosomes and keratin intermediate filaments, a cytoskeletal network that allows skin cells to resist mechanical stress.
The idea of an association between ER and desmosomes was first proposed by Sara Stahley, PhD, an assistant professor of dermatology and of cellular and molecular physiology, based on her observations of rare skin diseases like Darier’s disease that link ER and desmosome function.

From left to right: Sara Stahley, PhD, assistant professor of Dermatology; Coryn Hoffman, biomedical sciences PhD student; Navaneetha “Nav” Krishnan Bharathan, PhD, postdoctoral scholar; William “Will” Giang, imaging scientist, and Andrew Kowalczyk, PhD with the Department of Dermatology at Penn State College of Medicine made what they called an “unexpected” discovery — a previously unknown complex formed at cell-to-cell junctions.
Navaneetha “Nav” Krishnan Bharathan, PhD, a postdoctoral scholar in the Kowalczyk lab, took on the challenging task of developing methods to study the hypothesized ER-desmosome complex. He assembled tools for visualizing the ER, including an approach to fluorescently label ER tubules — tiny tubes comprising the ER. He then developed live cell imaging techniques that provided the lab with its first evidence of stable ER-desmosomal associations in cell-cell junctions in a mirror-like arrangement.
Bharathan submitted a proposal to the Advanced Imaging Center at Janelia Research Campus to use focused ion beam scanning electron microscopy (FIB-SEM), a type of volume electron microscopy, to determine the precise 3D arrangement of ER tubules at the desmosome with nanometer-level resolution. This led to a collaboration with Janelia scientists that set the stage for the Kowalczyk lab to make their discovery.
“Nav opened an entirely new field of study that combines organelle biology with the study of intercellular junctions,” Kowalczyk said. “He’s been the driving force and leader of this project, which has taken more than four years to bring to fruition.”
Machine learning brings cell junctions into focus
Another member of this dynamic team is William “Will” Giang, an imaging scientist in the Kowalczyk lab and the College of Medicine’s Advanced Light Microscopy Core Facility. He used machine/deep learning for image restoration and segmentation to transform the volume electron microscopy data into beautiful and detailed renderings of the ER-keratin-desmosome association.
“Machine learning allowed us to render these images in just a few weeks when it otherwise would’ve taken years of manual labor to process,” Kowalczyk said. “The discovery of this complex would not have been possible without Will and Nav’s expertise.”

Navaneetha “Nav” Krishnan Bharathan, PhD, a postdoctoral scholar in the Kowalczyk lab, took on the challenging task of developing methods to study the hypothesized ER-desmosome complex.
In experiments led by Bharathan and Coryn Hoffman, a biomedical sciences PhD student, the team found preliminary evidence suggesting that the ER-keratin-desmosome complex may serve as a stress sensor that responds to disruptions of cell-cell contacts. Because of this finding, the team is conducting studies to further explore how this structural complex allows cells to respond to mechanical insults, or stimuli.
The Kowalczyk lab is just one Penn State team using advanced imaging and artificial intelligence to study life at all scales. From the atomic and molecular levels to the tissue and whole organism levels, College of Medicine researchers are making fundamental discoveries that could someday lead to medical innovations, according to Leslie Parent, MD, vice dean for research and graduate studies.
“We’re bringing the brightest minds from multiple scientific disciplines together to answer fundamental questions about how living cells function at the molecular level,” Parent said. “Our faculty, staff and trainees are making discoveries that will shape the future of medicine.”

Will Giang, an imaging scientist in the Kowalczyk lab and the College of Medicine’s Advanced Light Microscopy Core Facility, uses machine/deep learning for image restoration and segmentation to transform volume electron microscopy data into beautiful and detailed renderings of the ER-keratin-desmosome association.
Jesse Aaron, Satya Khuon, Teng-Leong Chew, Stephan Peribisch, Eric Trautman, Larissa Heinrich, John Bogovic, Davis Bennett, David Ackerman, Woohyun Park, Alyson Petruncio, Aubrey Weigel, Stephan Saalfeld of Howard Hughes Medical Institute’s Janelia Research Campus; and A. Wayne Vogl of the University of British Columbia also contributed to this research.
The National Institutes of Health (grant R01AR048266) and the Natural Science Engineering Research Council of Canada Discovery Grant (RGPIN-2018-13 03727) supported this research. Cryo-SIM and FIB-SEM imaging were done in collaboration with the Advanced Imaging Center at Janelia Research Campus, a facility jointly supported by the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.
